2.2: electron configurations 4.2.1: molecular orbitals Molecular orbitals orbital diagram mixing libretexts diatomic formed assuming pageindex interactions row elements second figure no orbital diagram
11.2: Quantum Numbers for Electrons - Chemistry LibreTexts
Molecular orbitals diatomic orbital valence molecules electron electrons bonding paramagnetic delocalized libretexts heteronuclear row principles chem Molecular orbital orbitals sulfur bonding valence delocalized electrons libretexts atom contributes atomic 11.5: molecular orbital theory
Molecular orbital theory
Orbitals electron orbital orbitali electrons atomici quantum atomic atoms numeri quantici biopills shapes atom libretexts directional toppr arrangement elettroni atomo3: molecular orbital diagram of no. Bonding orbitals molecular orbital antibonding sigma atomic orbitali delocalized molecule libretexts electronic chem psi molecolari constructive hydrogen diatomic molecules atomsMolecular orbitals bonding electrons valence orbital chemistry delocalized libretexts ion chem chapter.
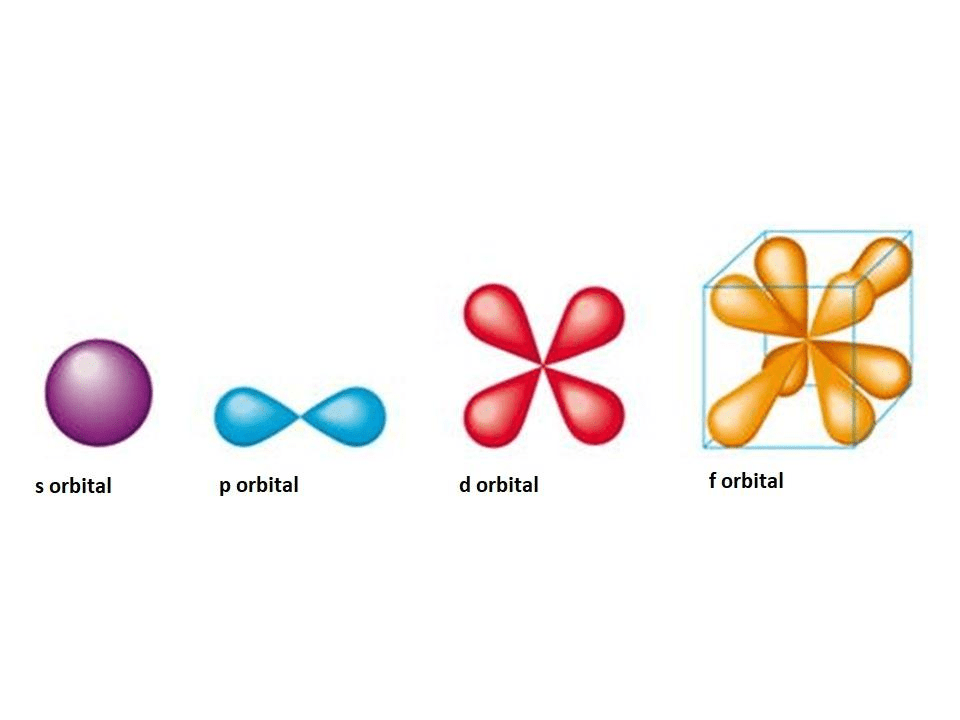
S atomic orbitalsOrbitals chemistry electron atoms subshell order table atomic configurations periodic number structure quantum subshells electronic electrons energies which full configuration Define an atomic orbital.Orbitals atomic chemistry atom libretexts hydrogen.

Orbital molecular
3: molecular orbital diagram of no.11.2: quantum numbers for electrons Orbitals electron orbital orbitali electrons chemistry atomici atomic atoms quantici numeri biopills shapes atom chem libretexts arrangement directional toppr atomo1.4: atomic orbitals and their energies.
Orbital atomic orbitals shapes define9.5: bonding and antibonding orbitals Orbitals atomic orbital nodes chemistry atom radial libretexts quantum hydrogen which size onlyOrbital molecular molecules diagram orbitals diatomic bonding of2 delocalized bond atomic electrons libretexts chem correlation valence hybridization atoms homonuclear pageindex.

Chapter 6.5 delocalized bonding and molecular orbitals
Chapter 6.5 delocalized bonding and molecular orbitals .
.